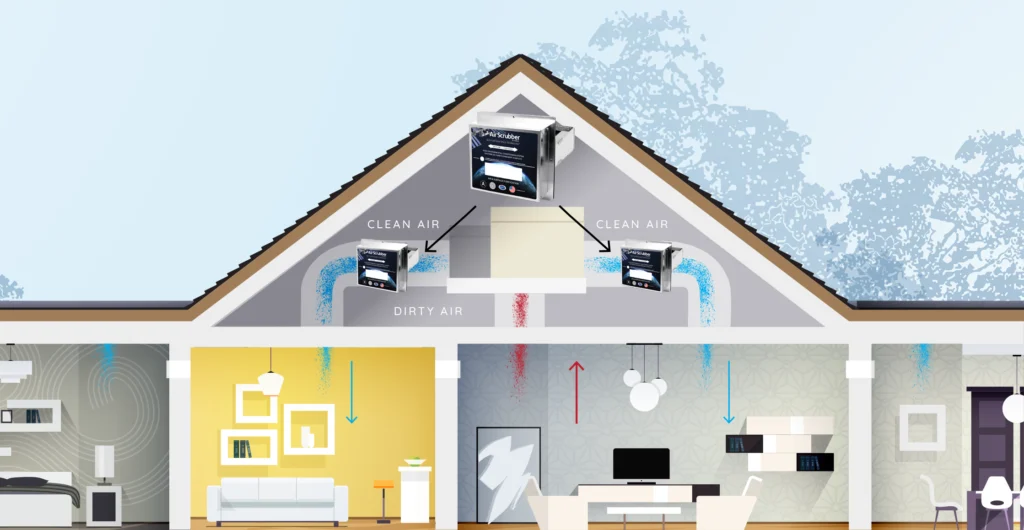
Air Scubber In Jacksonville, Pittsfield, Beardstown, And Surrounding Areas
ActivePure® Technology
Setting A New Standard In Indoor Air And Surface Purification
Frequently Asked Questions (Vol. 1 - 28.08.23)
ActivePure is an air and surface purification technology. It has been proven in both laboratory and field testing to quickly reduce bacteria, viruses, mold spores,1 and VOC gases2 in indoor spaces. A more complete list of tested pathogens can be found at activepure.com/scientific-proof. The ActivePure Medical Guardian has been cleared by the FDA as a Class II Medical Device.
ActivePure works by replicating the cleansing power of the sun; this is known as photolysis. Sunlight cleanses the air we breathe by creating hydrogen and oxygen-based molecules that inactivate viruses, bacteria, mold spores, and unwanted gases at the molecular level. ActivePure Technology creates and distributes these same molecules indoors. The molecules are released into the air of a room, traveling safely to quickly inactivate pathogens in the air and on surfaces.
ActivePure Technology produces molecules that fly through the air to inactivate pathogens in the air and on surfaces; imagine them scrubbing the air and surfaces clean. ActivePure is an active (as opposed to passive) purification method.
Distinguishing Activepure Technology From Other Technologies
Passive air cleaning technologies require that a contaminant be pulled into and pass through a mechanism in order to be eliminated from the air. For instance, a filter draws air through it to capture contaminants. Passive systems generally work slowly compared to active systems. Active purification technologies—such as ActivePure Technology— work by filling the air in a room with active molecules or energy — thereby going to the contaminant. With active technologies, contaminants can be inactivated quickly, even if they are not captured.
ActivePure is a purification technology primarily designed for use against bacteria, viruses, mold spores, and VOC gases. It has also been shown to be effective in reducing dust, smoke, pollen, dander, and other particulates, and is even more effective as such when used in combination with a HEPA filter. Some ActivePure units include a HEPA filter to optimize the impact on these particles.
Generally, we do not disagree with that, which is why we have combined HEPA filtration with ActivePure Technology in our ActivePure Medical Guardian, which has been cleared by the FDA, as a Class II Medical Device for use in healthcare settings. With ActivePure, HEPA filtration units can be materially more effective. Most importantly, ActivePure meets all CDC guidelines for emerging air disinfection technologies. This includes documented data under as-used conditions from multiple third-party sources. Moreover, devices with ActivePure Technology have been shown to meet the CDC recommended UL 2998 Environmental Claim Validation Procedure (ECVP) for Zero Ozone Emissions.

Please see each individual technology below.
- ActivePure is not an air filter such as HEPA or MERV 13.
- Filters are passive systems, so they must wait for airborne pathogens to come to them; consequently, they generally do not act quickly
- Filters do not always capture harmful particles; many of which are tiny and may pass through the filter
- Pathogens may not be inactivated by filters3; they are merely trapped
- Read more about filters at: blog.activepure.com/arrestance-cadr-merv/
- ActivePure is not bi-polar ionization.
- Bi-polar ionizers use electricity to produce ions; these ions impart an electrical charge to nearby particles, clumping them together and causing them to fall out of the air
- Bi-polar ionizers have not been cleared by the FDA and generally do not inactivate pathogens; their primary goal is to cause particles to fall out of the air and onto surfaces
- Particles that fall to a surface may contain harmful pathogens, and can easily be stirred up once more4
- ActivePure is not a ventilation solution.
- Ventilation is generally a good remedy to dilute pathogens; with some caveats
- Increasing ventilation can be expensive by increasing the amount of outdoor air needing to be heated or cooled; this increases energy costs and carbon footprint
- Outdoor air can also contain contaminants; bringing more outdoor air in at the wrong time of day can potentially increase particulate matter5
- Many older HVAC systems are not designed to materially increase ventilation (or filtration), as a result, increasing ventilation and filtration in indoor spaces may require the replacement of HVAC systems
- ActivePure is not UVGI purification.
- Robot-style Whole Room UV light can be effective as a one-time treatment, but has some key disadvantages
- Whole Room UV only treats what is directly exposed to the UV light6
- Whole Room UV can only be used in unoccupied rooms as it is harmful to the eyes and skin
- Whole room UV does nothing to help in occupied rooms if new pathogens are being introduced to the space
- Wall, duct, or ceiling-mounted UV lights must be shielded, making them a passive technology
- It is not easy to calibrate a UVGI system so that it has enough time to inactivate pathogens during the time they are exposed
- UVGI does not reduce unwanted gases7
- Read more about ActivePure vs. UVGI at: blog.activepure.com/indoor-air-ultraviolet-tech-2/
- ActivePure is not PCO technology.
- ActivePure is an advanced photocatalysis technology — not PCO
- Early generation PCO-type air cleaners can generate ozone and VOC gases8, potentially making them unsafe to use in indoor environments — ActivePure does not do this
- PCO is a passive technology
- ActivePure is not a chemical surface disinfectant.
- Surface disinfectants are chemical agents that can kill or eliminate microorganisms on surfaces
- Surface disinfectants introduce chemicals into the environments
- Most surface disinfectants do not protect against microbes re-introduced after application
Read more about ActivePure Technology vs. other methods at: blog.activepure.com/11-air-filtration-technologies/
Technology FAQs
Some PCO devices do that, ActivePure does not — ActivePure is not PCO. While the technologies are similar, ActivePure’s proprietary catalyst allows for the breakdown of VOC gases. And unlike some PCO devices, devices with ActivePure Technology have been shown under
CDC recommended UL 2998 procedures to meet zero ozone claim requirements.
According to an extensive study9 of ActivePure devices under field conditions, “In all cases, TiO2 levels were found to be below levels of concern.”
No, the CDC “does not provide recommendations for, or against, any manufacturer or product.” They advise consumers that many air cleaning technologies are “emerging.” Thus, consumers should “request testing data that quantitatively demonstrates a clear protective benefit and occupant safety under conditions consistent with the intended use.” Center for Disease Control. (2021). “Ventilation in Buildings.” cdc.gov/coronavirus/2019- ncov/community/ventilation.html. ActivePure (founded in 1924) has invested millions into research and testing of its products to provide customers with this data. And as stated above, ActivePure has been shown to meet CDC guidelines for emerging air disinfection technologies, including
UL 2998 zero ozone claim requirements.
Negative ionization’s dangers mostly stem from negative atomic ions, such as the hydrogen anion H−. Bi-polar ions that produce this aim to clump particles together. Some studies suggest that may cause particles to stick to the lungs.10 In contrast, superoxide and hydroxyl ions are negative molecular ions, created by sunshine, which deactivate germs directly rather than clumping them together.11
Hydroxyl radicals help clean the outdoor air every day; ActivePure Technology merely creates these molecules indoors too. Free radicals are different from hydroxyl radicals. Free radicals have been associated with unwanted health effects.
Every electronic device has the potential to produce a negligible amount of unintentional ozone. The California Air Resource Board (CARB) and UL 2998, require that air cleaning devices do not produce more than 0.05 ppm of ozone.12 CARB and UL have cleared every ActivePure device presented. The ActivePure Beyond Guardian Air, for instance, has been shown to produce a peak ozone level of 0.0008 ppm—less than 0.02% of CARB’s permissible limit.13
To read more about air purifier safety considerations, please visit blog.activepure.com/air-purifier-safety-concerns/
Efficacy FAQs
The Aerus Pure & Clean/Vollara Air & Surface Pro was shown to reduce airborne SARS-CoV-2 viral particles below the detectable limits in ONE minute in an enclosed space under laboratory conditions.14
Unaffiliated laboratories have completed extensive testing demonstrating that devices with ActivePure Technology—when used as tested—can eliminate up to 99.9% of viruses, bacteria, and mold spores in the air within minutes, and on surfaces within hours. For instance: the ActivePure/Aerus Medical Guardian eliminated 99.99% of Staphylococcus Epidermidis Gram-positive Bacteria, Aspergillus Niger fungal mold, and Bacillus Globigii bacterial mold within 60 minutes or less.15 Full list of tested pathogens is available at activepure.com/scientific-proof.
With over 70 products featuring ActivePure Technology, virtually any indoor space can be outfitted with a custom solution. We have run many tests over the years that validate the efficacy of ActivePure Technology in live field settings and encourage new clients to run such tests as well to ensure efficacy on site meets their expectations. ActivePure does have sensors that monitor results as well.
Every application is unique. We endeavor to custom design ActivePure to achieve desired outcomes in every installation. One extensive field study of ActivePure devices16 in a real-world school setting had this to say:
“It is reasonable to assume that: If used according to manufacturer’s recommendations and in combination with an appropriate cleaning program, the tested devices [ActivePure] will safely and continuously eliminate viral particles in air, reduce bacterial loads and minimize other ambient dusts.” 17
Economics & Environmental FAQs
Common guidance suggests increasing ventilation and filtration while only briefly acknowledging what kind of economic impact this might have on energy costs, existing infrastructure, and pathogen reduction outcomes. Building ventilation is a complicated field that requires balancing energy efficiency, temperature, humidity, indoor contaminants, and outdoor contaminants. Regarding the latter, bringing in outside air at the wrong time of day might increase contaminants indoors. ActivePure can provide sensors to monitor and optimize indoor air and the introduction of ventilated outdoor air.
Very quickly. Each induct unit can be installed in less than an hour by your in-house maintenance personnel.
Our portable units require no HVAC modifications at all and can begin operating immediately.
Very little. When used as directed, we recommend replacing ActivePure cells once a year; this is comparable to the maintenance of other cleaning technologies. HEPA filters and activated carbon filters in ActivePure units need replacement about once every 6 to 12 months.
All these replacements can be performed by in-house personnel in a matter of minutes.
ActivePure devices don’t require installing high-MERV filters which can strain the HVAC system and increase energy costs.
Also, most units use less than an amp of power. ActivePure, properly installed and monitored, may also allow for the reduction of heated and cooled outdoor air ventilation and on MERV 12-14 filters.
By installing a solution with a low-carbon footprint that does not leave residual surface chemicals, ActivePure Technology can be
an integral part of your organization’s energy, social, and environmental goals. With ActivePure, many clients reduce the ventilation and filtration costs, and attendant energy usage. Your commitment to employee/staff/customer/student peace of mind may also boost your reputation for good governance and social responsibility and reduce risks.
To learn more about ActivePure’s solutions for your organization, please contact your local representative or visit: airscrubberbyaerus.com/contactus/
CITATIONS
- ActivePure. (2023). “Proof Book” [Internal Compilation of Studies] Available under NDA.
- Lighthouse & ResInnova. (2021). “The School District of [Redacted] ActivePure Air System Study.” Lighthouse Environmental Infection Prevention.
- Hammond, A., et al. (April 2021). “Should homes and workplaces purchase portable air filters to reduce the transmission of SARS-CoV-2 and other respiratory infections? A systematic review.” PLoS ONE 16(4): e0251049. doi.org/10.1371/journal.pone.0251049
- (2021). “Do Ionic Air Purifiers Work?” Black-Mold-Guide.com. black-mold-guide.com/do-ionic-air-purifiers-work.html
- Lv, Yang, et al. (2017). “The Correlation between Indoor and Outdoor Particulate Matter of Different Building Types in Daqing, China” Procedia Engineering, 205(360-367) doi. org/10.1016/j.proeng.2017.10.002.
- U.S. Food & Drug Administration. (2021). “UV Lights and Lamps: Ultraviolet-C Radiation, Disinfection, and Coronavirus” https://www.fda.gov/medical-devices/coronavirus- covid-19-and-medical-devices/uv-lights-and-lamps-ultraviolet-c-radiation-disinfection-and-coronavirus
- U.S. Environmental Protection Agency. (2008). “Guide to Air Cleaners in the Home.” EPA.gov. epa.gov/sites/default/files/2014-07/documents/aircleaners.pdf
- Concordia University. (2015). “Certain air filters using photocatalytic oxidation have dangerous by-product, study shows: Dangerous by-products released by a filter already in commercial use.” ScienceDaily. sciencedaily.com/releases/2015/07/150715130835.htm
- Pennoni. (2022). “Report of Findings: Executive Summary ActivePure Technologies: Risk Assessment” [White Paper/Unpublished Lab Study]. Pennoni Associates Inc.
- Ali, Mohammed. (March 2009). “Measurements of electrodynamic effects on the deposition of MDI and DPI aerosols in a replica cast of human oral-pharyngeal-laryngeal airways.” Journal of Aerosol Medicine and Pulmonary Drug Delivery. 22(1):35-44. liebertpub.com/doi/10.1089/jamp.2007.0637
- Forster, P., et al. (2007). “Changes in Atmospheric Constituents and in Radiative Forcing. In: Climate Change “The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (Solomon, S., et al. Ed.) Cambridge University Press.
- California Air Resources Board. (2010). “California’s Regulation to Limit Ozone Emissions from Indoor Air Cleaning Devices.”ARB.CA.gov. ww2.arb.ca.gov/resources/fact- sheets/californias-regulation-limit-ozone-emissions-indoor-air-cleaning-devices
- Intertek (2013). “BGA Ozone Report.” Intertek.
- Lawrence, William S. & Peel, Jennifer E. (2022). “ActivePure Air Purifier Against Respiratory Pathogens” [Unpublished Lab Study] Available under NDA. University of Texas Medical Branch.
- ActivePure. (2023). “Proof Book” [Internal Compilation] Available under NDA.
- Pennoni. (2022). “Report of Findings: Executive Summary ActivePure Technologies: Risk Assessment” [White Paper/Unpublished Lab Study]. Pennoni Associates Inc.
- Devices tested in this study: Beyond Guardian Air, Pure & Clean, AP500, and Mid Range Balster.
- Pennoni. (2022). “Report of Findings: Executive Summary ActivePure Technologies: Risk Assessment” [White Paper/Unpublished Lab Study]. Pennoni Associates Inc.